-

FDA once again pushes back proposal to ban cancer-linked formaldehyde in hair relaxers
The Food and Drug Administration’s proposal to ban formaldehyde in hair care products has been pushed back once again, and the timeline for its release remains undetermined.
-

FDA OKs first menthol e-cigarettes, citing potential to help adult smokers
Friday’s action is the government’s strongest indication yet that switching to flavored vaping can reduce the harms traditional tobacco smoking.
-

The FDA reverses its ban on Juul e-cigarettes
Juul’s nicotine products have been allowed to stay in stores pending review of its application to sell them.
-

Panel rejects psychedelic drug MDMA as a PTSD treatment in possible setback for advocates
A first-of-a-kind proposal to begin using the mind-altering drug MDMA as a treatment for PTSD was roundly criticized Tuesday — a potentially major setback to psychedelic advocates who hope to win a landmark federal approval and bring the banned drugs into the medical mainstream.
-

Psychedelic drug MDMA faces questions as FDA considers approval for PTSD
Federal health regulators are set to review the first request to approve the mind-altering club drug MDMA as a treatment for PTSD. The Food and Drug Administration posted its review of the drug on Friday, raising questions about its effectiveness and potential risks, including heart problems.
-

Can yogurt reduce the risk of Type 2 diabetes?
The U.S. Food and Drug Administration recently said it’s OK for producers of yogurt to make that claim even though it’s based on limited evidence.
-

Bird flu is spreading to more farm animals. Are milk and eggs safe?
A bird flu outbreak in U.S. dairy cows has spread to more than two dozen herds in eight states. That comes weeks after the nation’s largest egg producer found the virus in its chickens. Health officials continue to stress that the risk to the public is low and that the U.S. food supply remains safe and stable.
-

FDA warns about lead contamination in more cinnamon products in the US
The Food and Drug Administration issued a safety warning Wednesday, saying it identified additional cinnamon products in the United States that are contaminated with lead.
-

Watch out for fake Ozempic shots that are being sold through legitimate drug supply sources
The U.S. Food and Drug Administration says it has seized “thousands of units” of counterfeit Ozempic, the diabetes drug widely used for weight loss.
-

Lead contamination in applesauce pouches may have been intentional, FDA says
The lead contamination in recalled cinnamon applesauce pouches that potentially poisoned at least 65 children may have been intentional, the Food and Drug Administration said on Friday.
-
After recalls and infections, experts say safer eyedrops will require new FDA powers
Repeated recalls of eyedrops are drawing new attention to the limited powers U.S. regulators have to oversee medical products made overseas. Unlike prescription drugs, eyedrops and other over-the-counter products don’t get preliminary review by the Food and Drug Administration. In the case of two eyedrop recalls earlier this year, FDA inspectors had never previously visited the plants in India. In...
-
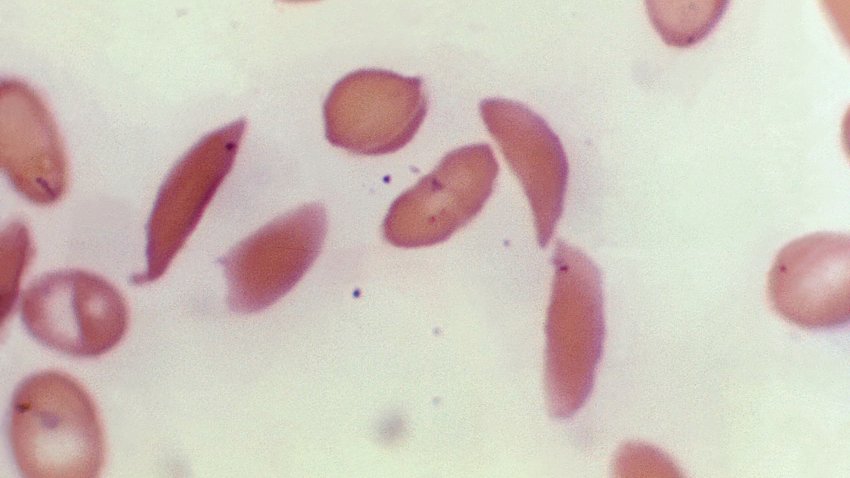
FDA approves two sickle cell disease therapies, including world's first gene-editing treatment
U.S. regulators have approved two gene therapies for sickle cell disease.
-
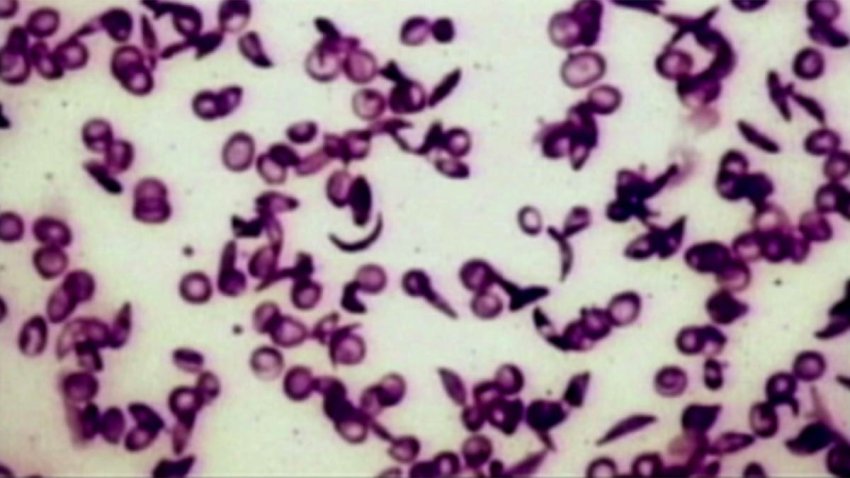
FDA approves groundbreaking treatments for sickle cell disease
One of the treatments uses CRISPR gene-editing technology.
-
Barefoot workers and cracked floors were found at a factory that made recalled eyedrops, FDA says
U.S. health inspectors found a host of sanitation and manufacturing problems at an Indian plant that recently recalled eyedrops sold in the U.S.
-

FDA screening US cinnamon imports after more kids sickened by lead-tainted applesauce
The U.S. Food and Drug Administration is screening imports of cinnamon from multiple countries for toxic lead after reports of illness in children who ate pouches of applesauce puree.
-

FDA warns consumers to stop using 26 eye drop products sold at CVS, Target and Rite Aid
The FDA is warning consumers to immediately stop using 26 over-the-counter eye drop products due to the “potential risk of eye infections that could result in partial vision loss or blindness.”
-
Novavax updated Covid vaccine wins FDA, CDC backing, paving way to reach Americans within days
Health officials see Novavax’s protein-based vaccine as a valuable alternative for people who don’t want to take messenger RNA shots from Pfizer and Moderna.
-
After US approval, Japan approves Leqembi, its first Alzheimer's drug
Japan’s health ministry has approved Leqembi, a drug for Alzheimer’s decease that was jointly developed by Japanese and U.S. pharmaceutical companies.
-

FDA skeptical of experimental ALS treatment pushed by patient advocates
NurOwn, a stem cell therapy, is at the center of a yearslong lobbying campaign by patients seeking access to experimental medicines. FDA regulators say the treatment hasn’t been shown to work.
-

FDA rejects first needle-free alternative to EpiPens, calling for additional research
The move came as a surprise: In May, an FDA advisory committee voted to recommend approval of the drug for children and adults.

